
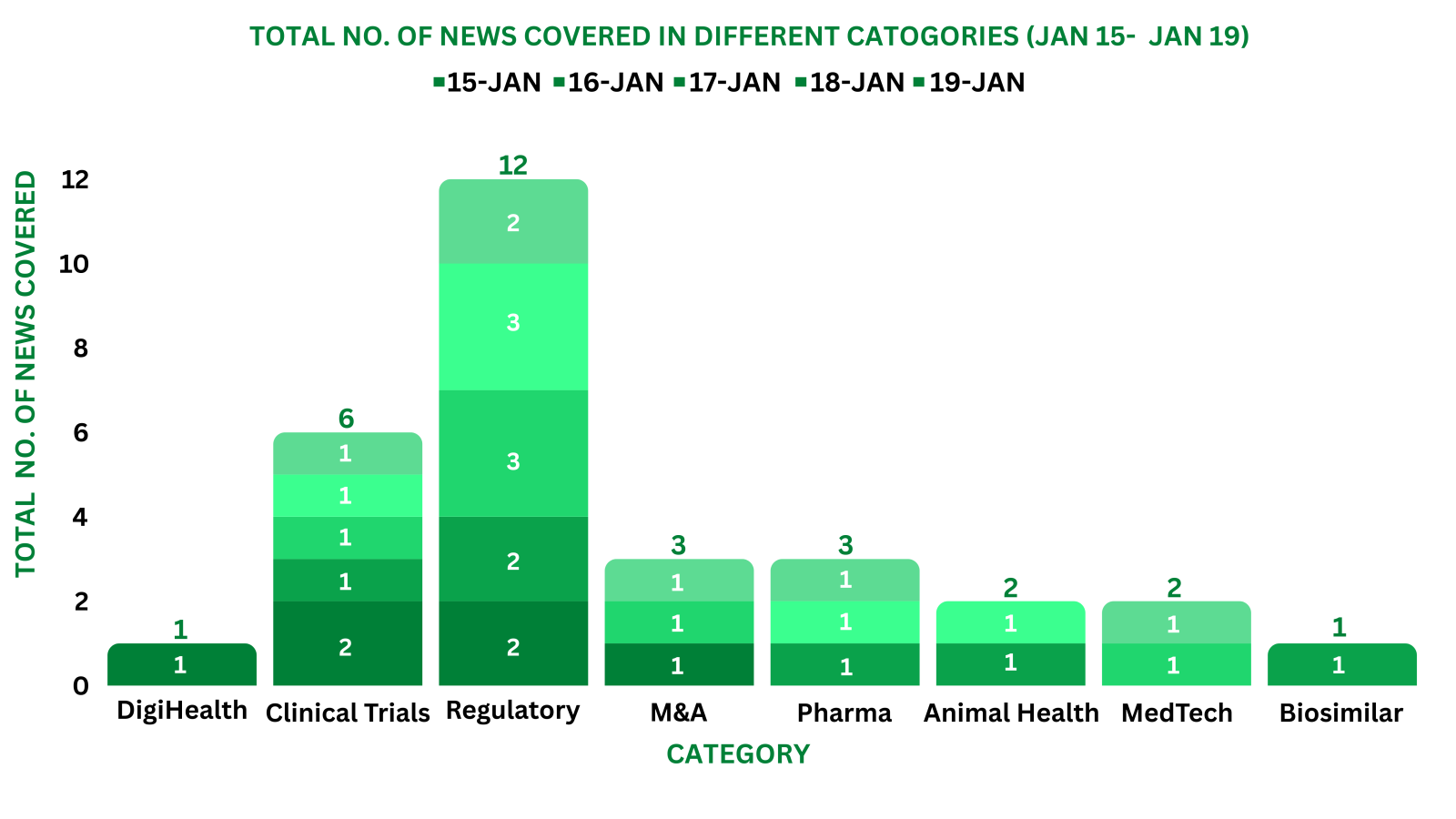
PharmaShots Weekly Snapshots (January 15 – January 19, 2024)
This week PharmaShots’ news was all about the updates on Regulatory, Clinical Trials,Pharma, Animal Health & MedTech. Check out our full report below:

The US FDA Approves Merck’s Keytruda + Chemoradiotherapy for Treating FIGO 2014 Stage III-IVA Cervical Cancer
Read More: Merck
MHRA Grants Approval to Santhera’s Agamree (Vamorolone) for Treating Duchenne Muscular Dystrophy
Read More: Santhera
The EC Grants Marketing Authorization to the Subcutaneous Formulation of Tecentriq for Multiple Cancers
Read More: Roche
The US FDA has Cleared the IND Application of InnoCare Pharma’s for ICP-248 to Treat Hematological Malignancies
Read More: InnoCare Pharma
The US FDA Updates Sanofi’s Dupixent Label for its Use in Atopic Dermatitis
Read More: Sanofi
The US FDA Clears Aruna Bio’s IND Application of AB126 for Neurological Indications
Read More: Aruna Bio
The EC Grants ODD to Precigen’s PRGN-2012 for Recurrent Respiratory Papillomatosis (RRP)
Read More: Precigen
The US FDA Approves Takeda’s Hyqvia as Maintenance Therapy for Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
Read More: Takeda
The Injection Formulation of Eisai’s Fycompa (perampanel) Receives MHLW’s Marketing Authorization for Epilepsy
Read More: Eisai
MHLW Grants Approval to argenx’s Vyvdura Injection for Generalized Myasthenia Gravis
Read More: argenx
Sensorion Obtains Approval to Conduct P-I/II Study of SENS-501 (OTOF-GT) for Hearing Impairment Across European Countries
Read More: Sensorion
The EPO Grants Patent to GH Research’s Mebufotenin (5-MeO-DMT) and its Salt Products for Major Depressive Disorder and Treatment-Resistant Depression
Read More: GH Research
Arcutis Biotherapeutics Highlights the P-III Resutls for Zoryve Cream for the Treatment of Atopic Dermatitis
Read More: Arcutis Biotherapeutics Date: Jan 15, 2023
Arcutis Biotherapeutics Reports Data from the STRATUM Study of Zoryve (Roflumilast) Topical Foam, 0.3% for Seborrheic Dermatitis
Read More: Arcutis Biotherapeutics Date: Jan 15, 2023
AM-Pharma Reports Initiation of P-II Study Investigating Ilofotase Alfa for Cardiac Surgery-Associated Renal Damage
Read More: AM-Pharma Date: Jan 16, 2023
Galderma to Highlight Results from the P-III (READY-3) Study of RelabotulinumtoxinA for Treating Frown Lines and Crow’s Feet at TOXINS 2024
Read More: Galderma Date: Jan 17, 2023
PeproMene Bio Reveals Results from the P-I Study of PMB-CT01 for Treating R/R B-Cell Acute Lymphoblastic Leukemia
Read More: PeproMene Bio
Coherus Presents Data from the P-II Study of Casdozokitug for Advanced or Metastatic Hepatocellular Carcinoma (uHCC)
Read More: Coherus

Andelyn Biosciences and Armatus Bio Collaborate to Manufacture Gene Therapy for Charcot-Marie-Tooth Type 1A (CMT1A)
Read More: Andelyn Biosciences & Armatus Bio
Xenetic Biosciences and University of Virginia Collaborate to Advance DNase-Based Oncology Platform
Read More: Xenetic Biosciences & University of Virginia
AbbVie and Enigma Biomedical Group (EBG-USA) Collaborate to Develop and Commercialize 4R Tau PET Imaging Biomarkers
Read More: AbbVie & Enigma Biomedical Group

Boan Biotech Reports Clinical Updates for BA6101 (biosimilar, Prolia) and BA1102 (biosimilar, Xgeva) to Treat Various Indications
Read More: Boan Biotech

For an Aggregate of $2.34M, Basilea Pharmaceutica Acquires Spexis’ Preclinical Antibiotics Program
Read More: Basilea Pharmaceutica
Vision Sensing Acquisition and Mediforum Sign a Definitive Merger Agreement
Read More: Vision Sensing Acquisition & Mediforum
Sun Pharma and Taro Pharmaceutical Sign a Definitive Merger Agreement
Read More: Sun Pharma & Taro Pharmaceutical

Delcath Systems Lanches Hepzato Kit in the US for Treating Metastatic Uveal Melanoma (mUM)
Read More: Delcath Systems
Abbott Conducts First Global Procedure in the Study of Volt PFA System for Abnormal Heart Rhythms
Read More: Abbott

Merck Animal Health Highlights the Updates from the Study Assessing the Mental Health and Well-Being of US Veterinarians
Read More: Merck Animal Health
ELIAS Animal Health Highlights Data from the (ECI-OSA-04) Study of ELIAS Cancer Immunotherapy (ECI) for Treating Dogs with Bone Cancer
Read More: ELIAS

The US FDA Approves Darmiyan’s BrainSee to Detect Alzheimer’s Dementia
Read More: Darmiyan
Related Post:- PharmaShots Weekly Snapshots (January 08 – January 12, 2024)
Tags

Shivani was a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She was covering news related to Product approvals, clinical trial results, and updates. We can be contacted at connect@pharmashots.com.














